
How to Use Chlorine Dioxide for Effective Water Treatment Solutions
Chlorine dioxide has emerged as a pivotal agent for effective water treatment solutions, particularly given the increasing stringent regulations on water quality across the globe. According to the World Health Organization, safe drinking water is essential for public health, highlighting the necessity for advanced purification methods. The growing prevalence of waterborne pathogens and contaminants necessitates innovative approaches to ensure that water systems remain both safe and reliable.
Recent studies from the Environmental Protection Agency indicate that chlorine dioxide water treatment can effectively eliminate a wide spectrum of harmful microorganisms, including bacteria, viruses, and protozoa, while maintaining water clarity and taste. In fact, research has demonstrated that chlorine dioxide is up to ten times more effective than traditional chlorine in certain scenarios, particularly in its ability to penetrate biofilms that often harbor resistant pathogens. With the global water crisis looming, these properties position chlorine dioxide as a cornerstone in developing efficient and sustainable water treatment technologies that meet the challenges of the modern age.
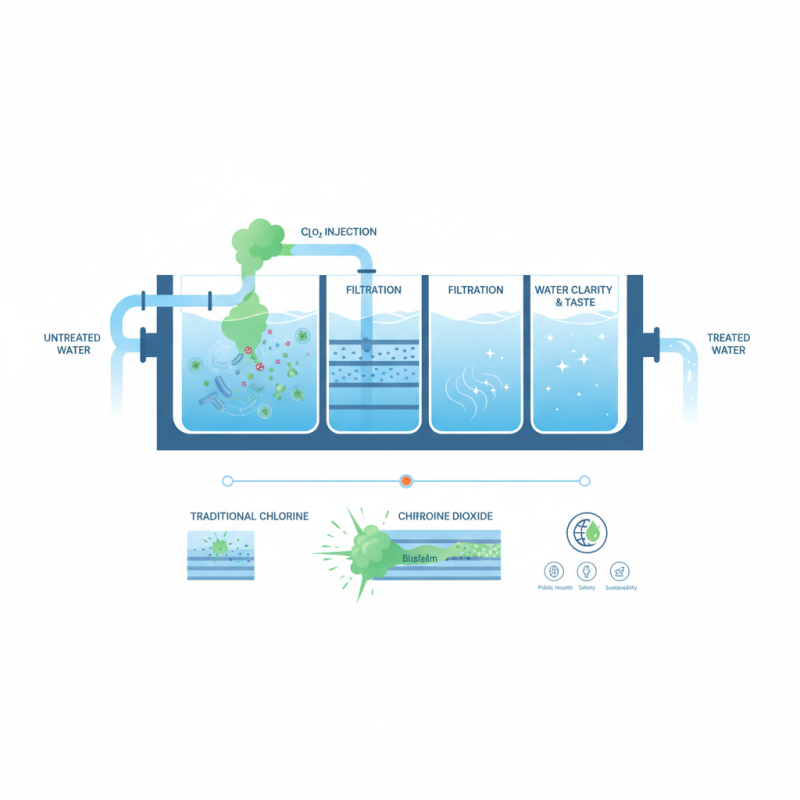
Overview of Chlorine Dioxide in Water Treatment Applications
Chlorine dioxide, a powerful oxidizing agent, has been widely adopted in water treatment processes due to its efficacy in disinfection and oxidation. Unlike chlorine, chlorine dioxide efficiently penetrates biofilms and biofouling, making it particularly effective in killing bacteria, viruses, and protozoa present in water systems. Its ability to function across a broad pH range and generate fewer undesirable by-products further enhances its appeal as a water treatment solution.
In various applications, chlorine dioxide is utilized to treat drinking water, wastewater, and industrial process water. It not only helps in the removal of organic compounds and chlorinated substances but also controls taste and odor issues commonly found in treated water. Additionally, its use in cooling towers and paper mills has been recognized for minimizing microbial growth and improving system efficiency. By implementing chlorine dioxide treatment, facilities can ensure compliance with health regulations while delivering high-quality water to consumers.
Chlorine Dioxide Applications in Water Treatment
Chemical Properties and Mechanisms of Chlorine Dioxide Action
Chlorine dioxide (ClO2) is a potent oxidizing agent that is used extensively for water treatment owing to its unique chemical properties. Unlike chlorine, chlorine dioxide functions effectively across a wide pH range, making it versatile for different water conditions. Its molecular structure allows for strong disinfection properties, effectively targeting a broad spectrum of pathogens, including bacteria, viruses, and protozoa. When ClO2 is introduced into water, it rapidly breaks down organic matter and contaminants through oxidation, leading to improved water quality.
The disinfection mechanism of chlorine dioxide primarily involves the selective oxidation of amino acids in microbial cells, disrupting crucial cellular functions. By targeting cell membranes and essential metabolic pathways, ClO2 damages the integrity of the microorganisms, rendering them inactive or nonviable. Additionally, chlorine dioxide's ability to form chlorinated by-products is significantly lower than that of traditional chlorine, resulting in fewer harmful residuals in treated water. This makes chlorine dioxide a favorable choice for achieving effective water treatment while minimizing negative environmental impacts.
How to Use Chlorine Dioxide for Effective Water Treatment Solutions - Chemical Properties and Mechanisms of Chlorine Dioxide Action
| Property | Value | Description |
|---|---|---|
| Chemical Formula | ClO2 | Chlorine dioxide's molecular structure. |
| Molecular Weight | 67.45 g/mol | Weight of one mole of chlorine dioxide. |
| Oxidation State | +4 | Oxidation state of chlorine in ClO2. |
| Solubility | 0.1 g in 100 mL of water | Chlorine dioxide's solubility in water. |
| pH Stability | 4-10 | Optimal pH range for chlorine dioxide effectiveness. |
| Half-Life | >1 hour in water | Stability duration in typical water conditions. |
| Disinfection Efficiency | >99.9% | Effectiveness in destroying bacteria and viruses. |
| Common Uses | Water treatment, odor control | Applications in various industries. |
Regulatory Standards and Safety Guidelines for Chlorine Dioxide Usage
Chlorine dioxide is recognized for its efficacy in water treatment, but its application must align with regulatory standards to ensure safety and compliance. The U.S. Environmental Protection Agency (EPA) classifies chlorine dioxide as a safe drinking water disinfectant, provided that its usage adheres to established concentration limits.
According to the EPA, the maximum residual disinfectant level for chlorine dioxide in drinking water is set at 0.8 mg/L. This regulation is crucial as excessive concentrations can lead to harmful by-products, which may affect human health and the environment.
Moreover, safety guidelines emphasize the importance of proper handling and application procedures for chlorine dioxide. The American National Standards Institute (ANSI) and the National Sanitation Foundation (NSF) highlight protocols to minimize exposure risks. Personnel involved in the treatment processes must undergo training to recognize potential hazards associated with chlorine dioxide, including its gaseous form, which can be detrimental if inhaled. Adhering to these guidelines not only ensures the safety of operators but also protects the communities reliant on treated water sources, as effective risk management plays a pivotal role in sustainable water treatment practices.
Comparative Efficacy: Chlorine Dioxide vs. Traditional Disinfectants
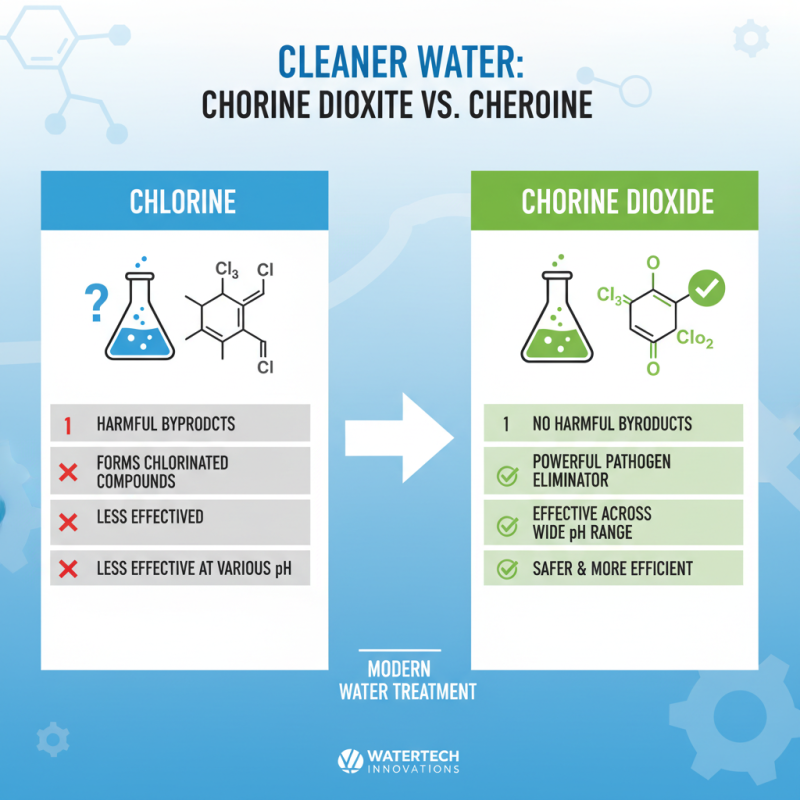
Chlorine dioxide has emerged as a powerful alternative to traditional disinfectants in water treatment applications. Unlike chlorine, which can produce harmful byproducts when reacting with organic materials, chlorine dioxide effectively eliminates bacteria, viruses, and other pathogens without generating chlorinated compounds. This quality makes it not only safer for human consumption but also more efficient in neutralizing a broader spectrum of microorganisms. Furthermore, chlorine dioxide operates effectively across a wider pH range, which expands its utility in varying water conditions.
When comparing chlorine dioxide to other disinfectants, one notable advantage is its lower required concentration for effective disinfecting. This allows for a more efficient treatment process with potentially reduced costs in chemical procurement. In addition, chlorine dioxide has a longer residual effect, providing ongoing protection against microbial regrowth in treated water systems.
Tips for using chlorine dioxide effectively include ensuring proper dosage based on water quality and pathogen load. Regular monitoring of water parameters, such as pH and temperature, can enhance disinfection efficiency. It’s also essential to maintain appropriate contact time between chlorine dioxide and the water to achieve optimal results. Implementing these practices can significantly enhance the effectiveness of water treatment solutions and ensure safe drinking water.
Case Studies: Successful Implementations of Chlorine Dioxide in Water Systems

Chlorine dioxide has emerged as a potent solution for water treatment in various applications, with numerous case studies showcasing its successful implementation in different water systems. One notable example occurred in a municipal water treatment facility where high levels of contaminants posed a challenge. By integrating chlorine dioxide into their treatment process, the facility was able to effectively reduce levels of bacteria and organic matter, resulting in significantly improved water quality. This transition not only enhanced public health safety but also minimized the formation of harmful by-products typically associated with other disinfection methods.
In another case study involving an industrial water system, chlorine dioxide was employed to control biofilm and improve the system's overall efficiency. The results demonstrated a remarkable reduction in microbial growth, which previously affected the operational performance of the system. The introduction of chlorine dioxide enabled the facility to maintain cleaner pipes and tanks, leading to increased system longevity and less frequent maintenance interventions. These examples illustrate the versatility and effectiveness of chlorine dioxide, establishing it as a reliable choice for water treatment in varying environments while emphasizing its benefits in safeguarding both public health and industrial infrastructure.
Related Posts
-

Why Industrial Water Treatment is Essential for Sustainable Practices
-

2025 How to Choose the Best Water Treatment Chemicals for Your Needs
-

What is Industrial Water Treatment and Why it Matters for Your Business
-
What is Water Analysis? Importance, Methods, and Key Benefits Explained
-

Why Water Analysis is Crucial for Your Health and Safety: Key Insights Explained
-

Top 10 Must-Have Water Equipment for Every Home and Business
Ready to talk about water treatment?
Contact us
Please complete this form and we will be in touch